| Summary |
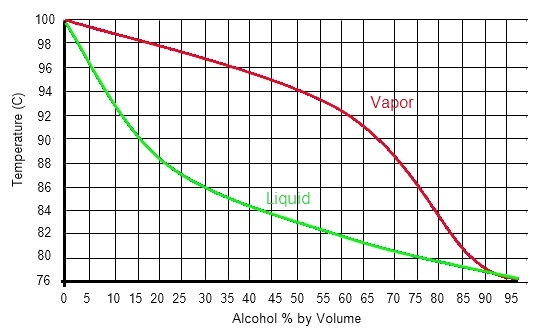
| When you heat up a mixture of liquids, the more volitile components will tend to come off first. There is a bit of overlap (so it is never pure), but generally we can seperate the ethanol from the water and other impurities present. The more alcohol in the liquid, the more alcohol will be in the vapour, so multiple distillations allow us to increase the strength & purity right up to 96.5% |

| http://homedistiller.org This page last modified |