Basic Data for Ethanol-Water Binary Mix
Physical Properties
| Value | Units | Ethanol | Water | Reference
| Liquid density | g/mL | 0.789 | 1.000 | Perry 3.2
| Vapour density @ 90C | g/mL | 0.0015 | 0.001 | PV=nRT
| Molecular weight | g/mol | 46.0634 | 18.0152 | Perry 3.2
| Liquid Heat Capacity | J/gK | 2.845 | 4.184 | Perry 3-183
| Heat of Vapourisation | J/g | 855 | 2260 | Perry 3-178
| Vapour Pressure @ 90C | torr | 1187 | 525 | Perry 13-4
| Liquid Viscosity | kg/ms | 0.00037 | 0.00032 | Perry 3-252
| Vapour Viscosity | kg/ms | 108 x 10-7 | 125 x 10-7 | Perry 3-311
| Surface Tension @ 20C | mN/m | 22.39 | 72.75 | Kay & Laby
| Vapour Diffusivity ethanol/water | m2/s | ? | ?
| Vapour Diffusivity ethanol/air | m2/s | 102 x 10-7 | Perry 3-319
| Liquid Diffusivity ethanol/water | m2/s | 128 x 10-11 | Perry 3-319
| | |||
Perry = Perry's Chemical Engineers' Handbook, 6th Ed, 1987, Robert H. Perry & Don Green
Kaye & Laby = Tables of Physical & Chemical Constants, Kaye & Laby, 16th Ed, 1995
Vapour Pressure
log (P_sat) = A - B/(T+C) : P_sat [torr], T [C]torr x 133.22 = Pa
Perry 13-4
| Species | A | B | C |
| Ethanol | 8.1122 | 1592.864 | 226.184 |
| Water | 8.07131 | 1730.63 | 233.426 |
Heat of Vapourisation
Hv = Ho * ( (1-T/Tc)/(1-To/Tc) ) ^ (A +B(1-T/Tc))| Value | Ethanol | Water |
| Ho | 38743.84 | 40799.86 |
| Tc | 513.9 | 647.3 |
| A | 0.32 | 0.19 |
| B | -0.14 | 0.21 |
Surface Tension
st [mN/m] = 24.05 - 0.0832 x T [oC] in range 10-70 oCEquilibrium Data
The molar data is from Perry 13-12, I've done the conversions to mass fraction and volume fraction, using the density and molecular weight data above.Temperature |
Mole Fraction |
Mass Fraction (g/g) |
Volume Fraction (mL/mL) |
|||
(C) |
Liquid (x) |
Vapour (y) |
Liquid (x) |
Vapour (y) |
Liquid (x) |
Vapour (y) |
95.5 |
0.019 |
0.170 |
0.0472 |
0.3437 |
0.0591 |
0.3990 |
89 |
0.072 |
0.389 |
0.1657 |
0.6196 |
0.2012 |
0.6736 |
86.7 |
0.097 |
0.438 |
0.2147 |
0.6654 |
0.2573 |
0.7160 |
85.3 |
0.124 |
0.470 |
0.2654 |
0.6943 |
0.3141 |
0.7422 |
84.1 |
0.166 |
0.509 |
0.3374 |
0.7260 |
0.3923 |
0.7705 |
82.7 |
0.234 |
0.545 |
0.4381 |
0.7535 |
0.4971 |
0.7948 |
82.3 |
0.261 |
0.558 |
0.4743 |
0.7635 |
0.5334 |
0.8036 |
81.5 |
0.327 |
0.583 |
0.5544 |
0.7811 |
0.6119 |
0.8189 |
80.7 |
0.397 |
0.612 |
0.6269 |
0.8014 |
0.6804 |
0.8365 |
79.8 |
0.508 |
0.656 |
0.7252 |
0.8301 |
0.7698 |
0.8609 |
79.7 |
0.520 |
0.660 |
0.7346 |
0.8322 |
0.7782 |
0.8628 |
79.3 |
0.573 |
0.684 |
0.7745 |
0.8470 |
0.8132 |
0.8753 |
78.74 |
0.676 |
0.739 |
0.8423 |
0.8784 |
0.8713 |
0.9015 |
78.24 |
0.747 |
0.782 |
0.8831 |
0.9014 |
0.9055 |
0.9206 |
78.15 |
0.894 |
0.894 |
0.9558 |
0.9558 |
0.9648 |
0.9648 |
Correlations
Converting these to something useful...
Vapour-Liquid Equilibrium
%vap = -94.7613*x ^ 8 + 450.932 * x ^ 7 - 901.175 * x ^ 6 + 985.803 * x ^ 5 - 644.997 * x ^ 4 + 259.985 * x ^ 3 - 64.5050 * x ^ 2 + 9.71706 * x
where %vap = % of ethanol in vapour if condensed (mL per mL), and x = % of ethanol in liquid (mL per mL).
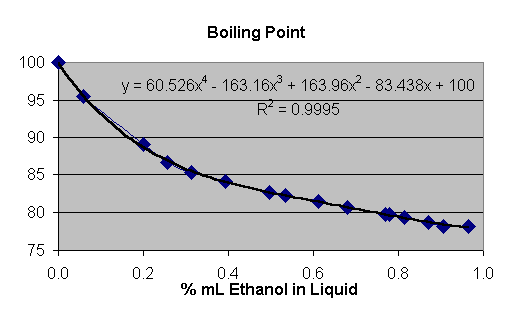
Temperature
T (in C) = 60.526 * %liq ^ 4 - 163.16 * %liq ^ 3 + 163.96 * %liq ^ 2 - 83.438 * %liq + 100
| http://homedistiller.org This page last modified |